Quote:
Originally Posted by BlackCrayon
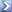
i'd sooner try crack that meth. meth is just some mixture of nasty ass chemicals.
|
Well.....
This article is about the free base and salts of methamphetamine. "Meth" and "crystal meth" redirect here. For other uses, see Meth (disambiguation)
Methamphetamine
An image of the methamphetamine compound
Ball-and-stick model of the methamphetamine molecule
Systematic (IUPAC) name
N-methyl-1-phenylpropan-2-amine
Clinical data
Pronunciation /ˌmɛθæmˈfɛtəmiːn/
Trade names Desoxyn
AHFS/Drugs.com monograph
Licence data
US FDA: Methamphetamine
Pregnancy
category
US: C (Risk not ruled out)
Legal status
AU: S8 (Controlled)
CA: Schedule I
DE: Anlage II
NZ: Class A
UK: Class A
US: Schedule II
UN: Psychotropic Schedule II
℞ (Prescription only)
Dependence
liability Physical: none
Psychological: high
Addiction
liability Very high
Routes of
administration Medical: oral (ingestion), intravenous[1]
Recreational: oral, intravenous, intramuscular, subcutaneous, smoke inhalation, insufflation, rectal, vaginal
Pharmacokinetic data
Bioavailability Oral: 70%[2]
IV: 100%[2]
Protein binding Varies widely[3]
Metabolism CYP2D6,[4] DBH,[5] FMO3,[6] XM-ligase,[7] and ACGNAT[8]
Onset of action Rapid[9]
Biological half-life 5â??30 hours[10]
Duration of action 10â??20 hours[9]
Excretion Primarily renal
Identifiers
CAS Number 537-46-2 Yes
ATC code N06BA03
PubChem CID 1206
IUPHAR/BPS 4803
DrugBank DB01577 Yes
ChemSpider 1169 Yes
UNII 44RAL3456C Yes
KEGG D08187 Yes
ChEBI CHEBI:6809 Yes
ChEMBL CHEMBL1201201 Yes
Synonyms N-methylamphetamine, N,α-dimethylphenethylamine, desoxyephedrine
Chemical data
Formula C10H15N
Molar mass 149.24 g·mol−1
Chirality 1:1 mixture (racemate)
SMILES[show]
InChI[show]
Physical data
Melting point 3 °C (37 °F) (predicted)[11]
Boiling point 212 °C (414 °F) at 760 mmHg[12]
(verify)
Methamphetamine[note 1] (contracted from N-methylamphetamine) is a strong central nervous system (CNS) stimulant that is mainly used as a recreational drug. Methamphetamine hydrochloride is approved by the United States Food and Drug Administration (USFDA) under the trade name Desoxyn for attention deficit hyperactivity disorder and obesity in adults and children, and is sometimes prescribed off label for narcolepsy and idiopathic hypersomnia. It is rarely prescribed due to concerns involving human neurotoxicity and its high potential for recreational use, among other concerns, as well as the availability of safer substitute drugs with comparable treatment efficacy. Methamphetamine exists as two enantiomers: dextromethamphetamine and levomethamphetamine.[note 2] Methamphetamine properly refers to a specific chemical, the racemic free base, which is an equal mixture of levomethamphetamine and dextromethamphetamine in their pure amine forms. Dextromethamphetamine is a much stronger central stimulant than levomethamphetamine. Both enantiomers are neurotoxic and addictive.
Both methamphetamine and dextromethamphetamine are illicitly trafficked and sold owing to their potential for recreational use as an aphrodisiac and euphoriant. The highest prevalence of illegal methamphetamine use occurs in parts of Asia, Oceania, and in the United States, where racemic methamphetamine, levomethamphetamine, and dextromethamphetamine are classified as schedule II controlled substances. Levomethamphetamine is available as an over-the-counter drug for use as an inhaled nasal decongestant in the United States.[note 3] Internationally, the production, distribution, sale, and possession of methamphetamine is restricted or banned in many countries, due to its placement in schedule II of the United Nations Convention on Psychotropic Substances treaty. While dextromethamphetamine is a more potent drug, racemic methamphetamine is sometimes illicitly produced due to the relative ease of synthesis and limited availability of chemical precursors.
In low doses, methamphetamine can elevate mood, increase alertness, concentration and energy in fatigued individuals, reduce appetite and promote (initial) weight loss. At higher doses, it can induce psychosis, rhabdomyolysis, seizures and cerebral hemorrhage. Chronic high-dose use can precipitate unpredictable and rapid mood swings, prominent delusions and violent behavior. Recreationally, methamphetamine's ability to increase energy has been reported to lift mood and increase sexual desire to such an extent that users are able to engage in sexual activity continuously for several days.[18] Methamphetamine is known to have a high addiction liability (i.e. compulsive methamphetamine use) and dependence liability (i.e. withdrawal symptoms occur when methamphetamine use ceases). Heavy recreational use of methamphetamine may lead to a post-acute-withdrawal syndrome, which can persist for months beyond the typical withdrawal period. Unlike amphetamine, methamphetamine is neurotoxic to humans, damaging both dopamine and serotonin neurons in the CNS.[19][20][21] This damage includes adverse changes in brain structure and function, such as reductions in grey matter volume in several brain regions and adverse changes in markers of metabolic integrity.[21]
Methamphetamine belongs to the substituted phenethylamine and substituted amphetamine chemical classes. It is related to the other dimethylphenethylamines as a positional isomer of these compounds, which share the common chemical formula: C10H15N1.